Science Behind Cord Blood.
Researchers continue to conduct cutting-edge clinical trials in areas such as:
In the late 1980s, doctors began treating blood cancers and leukemia with hematopoietic stem cells found through cord blood research . Up until then, patients could only utilize bone marrow stem cells. There were many cases where bone marrow treatment failed or a bone marrow match could not be found. Researchers continue to explore new applications for the use of cord blood stem cells around the world. Today, over 80 diseases have been treated with cord blood stem cells because of ongoing cord blood research and clinical trials! The FDA regularly reviews the results of clinical trials for new treatments.
- Early hearing loss in infants
- Cerebral Palsy
- Autism
- Congenital Pediatric Disorders
Cord Blood Research & Clinical Trials

Featured Advanced Cell Therapy Trial: Expanded Access Cord Blood Therapy for Autism and Cerebral Palsy

Duke University Medical Center has received permission from the FDA to offer cord blood therapy for conditions like autism spectrum disorder and cerebral palsy under an expanded access clinical trial. This protocol establishes an umbrella clinical trial NCT03327467 registered on 31 Oct. 2017 which enables children who have these neurological disorders to receive therapy with their own cord blood or cord blood from a sibling, regardless of whether they qualify for a targeted clinical trial.
The registration of this clinical trial is a watershed moment, opening the door for many children who are afflicted with an acquired neurological disorder to travel to Duke University for cord blood therapy, provided they have a suffienctly matching cord blood unit in a family bank. Sibling therapy only requires a partial match, not a perfect match. This expanded access protocol is a triple win for patients, family cord blood banks, and Duke University Medical Center.
The FDA may grant permission for expedited access (aka expanded access) under one of four designation pathways. In order to be considered for the FDA's expedited access prorgram, the new therapy must treat a “serious condition” that that has substantial impact on day-to-day functioning. In addition, there must be an “unmet medical need” because the condition is not addressed adequately by therapeutic alternatives.
The Breakthrough Therapy designation for expedited access is based on the results of phase 2 clinical trials. Duke University has conducted multiple clinical trials investigating the use of both autologous and allogeneic umbilical cord blood (UCB) in the treatment of cerebral palsy (NCT01147653, NCT02599207) and autism spectrum disorder (NCT02847182). As stated in the new open access clinical trial, “The use of (UCB) in this fashion is based on safety and efficacy data from prior and ongoing clinical trials at Duke University Medical Center in over 700 patients with these diagnoses infused with autologous or sibling UCB over the past decade.”
In the United States, the Centers for Disease Control and Prevention reports that the prevalence of cerebral palsy is 1 in 323 children (0.3%) and the prevalence of autism spectrum disorder is 1 in 68 children (1.5%). However, in order to be eligible to participate in the new clinical trial, patients must have their own or a sibling’s cord blood preserved in a family bank. To date, the only study that has examined the prevalence of medical conditions among families with privately stored cord blood is a recent publication that surveyed clients of Cord Blood Registry® (CBR®). The authors found that, out of 94,803 respondent families, 4.23% reported at least one child with an indication for regenerative therapy with cord blood. For conditions similar to autism spectrum disorder and cerebral palsy, the combined prevalence was 2.18%.
Worldwide, there are projected to be tens of thousands of children who are eligible to take advantage of this new treatment pathway. In the United States alone, there are over a million cord blood units in family storage, so that if 2% of the inventory corresponds to children with eligible conditions, that potentially translates into 20,000 patients.
Breaking News: The Cord Blood Association (CBA) announces 13 Nov. 2017 they are seeking funds to convert this protocol into a multi-center clinical trial administered by CBA and Duke.
Stem cells offer hope for autism
 READ MORE
READ MOREGracie Gregory smiles beneath her brilliant blue eyes. She's sitting on her mother's lap, next to her older sister, Ryleigh, who boasts about Gracie being "very sweet and kind." It wasn't always so. Just a couple years ago, Ryleigh, 11, was scared of her sister when she'd throw tantrums and screaming fits. "She would've fought and kicked," Ryleigh says, noting that it wouldn't have been possible to sit like this next to Gracie. Why was she scared of her sister? "Because of the kicking." Gracie, 7, interrupts: "I don't even remember it." "We do," says her mother, Gina Gregory. Gracie has autism, a condition that affected nearly every aspect of her family's life after she was diagnosed at 2. But a new study is offering hope for the Gregorys and families like them. Gracie was one of 25 children who took part in the first-of-its-kind study at Duke University in Durham, North Carolina. The goal: to see whether a transfusion of their own umbilical cord blood containing rare stem cells could help treat their autism. The results were impressive: More than two-thirds of the children showed reported improvements.
A larger second trial is underway, one its researchers hope will lead to long-term treatment for children with autism. Skeptics say there are too many unanswered questions to get excited. Even Duke researchers acknowledge as much. The initial trial, published Wednesday in the journal Stem Cells Translational Medicine, was a safety study, not a controlled, double-blind study with definitive proof of positive results. This study was open-label, meaning everyone -- the doctors and the families -- knew that the therapy was being administered. But for the Gregorys, the change in their daughter has been monumental.
Umbilical cord blood transplant linked to lower relapse in high-risk leukemia patients
READ MOREStudy compares ‘alternative’ donor source vs. traditional transplantation of blood stem cells from an adult, unrelated donor
Heart failure could be treated using umbilical cord stem cells
READ MOREUsing stem cells derived from the umbilical cord, researchers have improved the heart muscle and function of heart failure patients, paving the way for noninvasive therapies.
Bringing opportunity to CNY!
At Upstate Cord Blood Bank we offer families the option to donate their cord blood for patients in need of a life-saving transplant. If the collected cord blood does not contain enough stem cells to meet the standards to be clinically useful to a patient, families can choose to donate the cord blood to researchers seeking to advance new treatments. Therefore, these invaluable stem cells will not go to waste! This is especially beneficial for studies seeking to advance new treatments for cancer and chronic diseases. This makes the Upstate Cord Blood Bank in Syracuse, New York a valuable resource for patients, physicians, transplant centers, and researchers across the US and around the world.
The Bank operates under strict guidelines and protocols established by state and federal health organizations for quality assurance. This includes NYSDOH, FDA, FACT, AABB, CLIA, and CAP. Regular inspections and testing are done to ensure that our facility remains a safe haven for banked cord blood. Above all, this makes it possible to work with the regional hospitals and providers to enable mothers in the Central New York area the opportunity to donate their cord blood to the public bank.
The Science Behind Cord Blood Usage
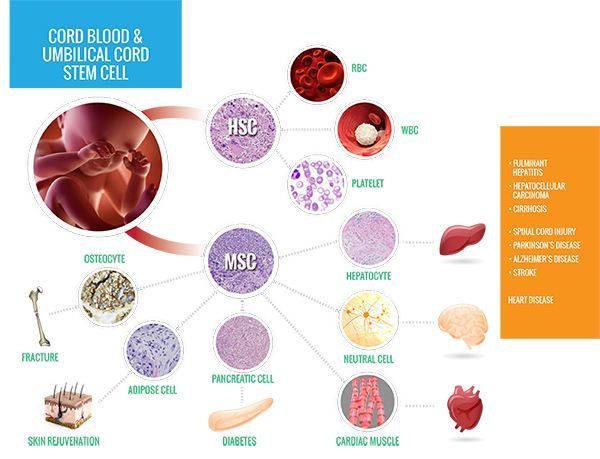
When patients are in need of a lifesaving stem cell transplant, there are several reasons why a doctor might choose cord blood:
Cord blood is only one of three sources of blood-forming cells used in transplants. These cells, known as hematopoietic stem cells (HSCs), are also found in bone marrow and peripheral blood stem cells.
HSCs are capable of renewing themselves into a variety of different cell types based on the treatment needs of the patient receiving the transplant. Because the healthy HSCs are extracted from cord blood at an early development stage, this gives the cells an even greater ability to self-replicate, and they are less likely to be rejected by the patient’s immune system.
When the cord blood arrives at our facility, we will run tests and begin processing it for long-term storage. During our process, we use highly specialized equipment creating a closed system to eliminate contamination while extracting the HSCs from the cord blood. The HSCs are secured in a vacuum-sealed overwrap bag made of TeflonTM and then placed into a protective metal receptacle to be stored in our state-of-the-art cryogenic tanks.
Each cord blood unit (CBU) is recorded in our local database using a unique identification number that keeps identities anonymous, but makes the unit easily retrievable when needed for a life-saving transplant. The donated CBU will also be uploaded to an international registry database to be made available to transplant centers all over the world for patients in need of a life-saving transplant.
Cord blood is being used for both children and adult patients. However, it is more likely that cord blood will be used in transplants for children. This is due to the fact that each CBU has a limited number of HSCs. Smaller patients will usually receive enough HSCs from one CBU, but fully grown patients will sometimes need to receive two or more combined CBUs for treatment.
- Cord blood does not have to be as closely matched to a patient as a bone marrow or adult donor. This is a good option for those patients that are difficult to match.
- Cord blood units are quickly available for transplantation as they are in storage and ready for use.
- Studies have found that graft-versus-host-disease (GVHD) is less common and less severe after a cord blood transplant than after a transplant using peripheral blood stem cells.
Cord blood and stem cells are in high demand, not only for immediate use as a transplant option for treatment of many diseases, but for continued advancement of research that is essential for the future of life-saving treatments. If you would like to partner with us to advance cord blood research, please call Upstate Cord Blood Bank at (315) 492-2600 today.



